Merging Iridium-Catalyzed Stereoselective Coupling from Alcohols with Organocatalytic Functionalization at the Aldehyde Oxidation Level
Résumé
We report the selective merging between a Krische-type iridium-catalyzed CC coupling and an organocatalyzed oxa-Michael addition. Such a general strategy triggers the direct double functionalization of alcohols through a complex multicatalytic pathway merging the two catalytic cycles in one. The iridium catalyst dehydrogenates the alcohol to an aldehyde which is intercepted in an organocatalyzed functionalization. Meanwhile, the generated iridium hydride activates a pro-nucleophile to subsequently create the final chiral alcohol through a stereoselective CC coupling on the functionalized aldehyde. The disclosed multicatalytic reaction, fulfilling all the principles of redox economy, enables the stereoselective preparation of complex tetrahydropyrans, scaffolds present in numerous natural products and featuring up to three controlled stereogenic centers.
Fichier principal
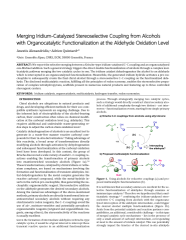 Ir Organocatalysis cascade without highlight.pdf (1010.66 Ko)
Télécharger le fichier
Ir Organocatalysis cascade without highlight.pdf (1010.66 Ko)
Télécharger le fichier
| Origine | Fichiers produits par l'(les) auteur(s) |
|---|